Neosoma Announces Receiving FDA 510(k) Clearance for Brain Mets
PR Newswire
GROTON, Mass., Dec. 23, 2025
Neosoma, a best-in-class provider of AI-based brain tumor analysis medical software, receives FDA 510(k) clearance for its Brain Mets product. This clearance ushers in a new era in the assessment of brain metastases, providing novel tumor insights to physicians and researchers in near-real-time.
GROTON, Mass., Dec. 23, 2025 /PRNewswire-PRWeb/ -- Neosoma, Inc. is excited to announce that it has received FDA 510(k) clearance for its Brain Mets software medical device. This clearance ushers in a new era in the assessment of brain metastases, integrating directly into Neosoma's unique, best-in-class tumor analysis cloud platform.
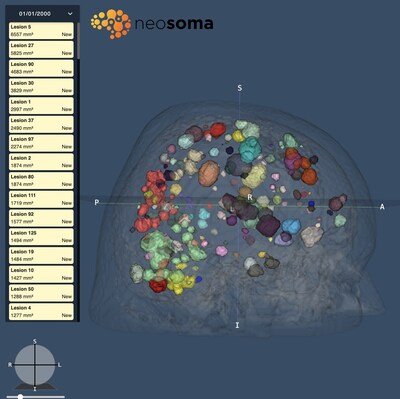
Brain metastases are the most common form of brain cancer, impacting up to 40% of all adult cancer patients, a significantly larger population than primary brain tumors. These cases are challenging to manage, typically involving numerous lesions of varying sizes, requiring frequent visual assessment of lesion size and count from MRIs to determine response to treatment.
Furthermore, the majority of these patients will undergo radiation treatment, the planning process for which involves manual hand-contouring of each lesion to be treated, which is itself a time-consuming process.
Neosoma's dedicated neuro-oncology software addresses these needs, expediting lesion identification, measurement, tracking, and target (GTV) contouring, all in a single cloud-based, highly-secure, PACS-integrated solution.
Jona Hattangadi-Gluth, MD, Professor at UC San Diego School of Medicine and Chief of the CNS Tumor Service in Radiation Oncology at Moores Cancer Center, said, "Brain metastases are common and complex, especially with the myriad of targeted systemic treatments and radiotherapy options. This technology will directly impact how we detect and surveil brain metastases after therapy, and allow us to quantify and measure response to novel therapeutics."
Neosoma Brain Mets was developed and rigorously validated to assist the multidisciplinary team, supporting radiology, radiation oncology, medical oncology, and surgical workflows, as well as tumor board decision-making.
For example, individual lesions are automatically quantified and identified longitudinally from routinely-acquired MRI sequences, requiring no special acquisition protocols, dramatically expediting the assessment process. Neosoma's technology auto-contours target (GTV) volumes, integrating seamlessly into radiation oncology and surgical planning software. There is no limit to the number of lesions that Neosoma Brain Mets can identify, contour, and track.
Neosoma's solution is the only clinically-available platform dedicated to neuro-oncology that combines advanced AI image analysis technology with a full-featured, interactive web-based user interface and patient imaging database.
Rupesh Kotecha, MD, Professor at FIU Herbert Wertheim College of Medicine and Chief of Radiosurgery and Director of CNS Metastasis of the Department of Radiation Oncology at Miami Cancer Institute, said, "The FDA clearance of this software to detect and segment brain metastasis marks meaningful progress towards more efficient and objective imaging in neuro-oncology. I look forward to seeing these tools being integrated across radiology, radiation oncology, and neuro-oncology workflows to support clinical decision-making and research."
Neosoma's solutions have been adopted by thought-leading clinicians and researchers across a number of the world's preeminent cancer centers. This important expansion of the company's integrated software suite for neuro-oncology will drive significant value for physicians, researchers, and, most importantly, patients.
Media Contact
Ken Kolodziej, Neosoma, Inc., 1 6178884080, ken.kolodziej@neosomainc.com, https://neosomainc.com
![]() View original content to download multimedia:https://www.prweb.com/releases/neosoma-announces-receiving-fda-510k-clearance-for-brain-mets-302648928.html
View original content to download multimedia:https://www.prweb.com/releases/neosoma-announces-receiving-fda-510k-clearance-for-brain-mets-302648928.html
SOURCE Neosoma, Inc.